HOME > Research Activities > Research Updates >
Confirming the Improvement of Hydrogen Solubility through the Mixing of Metallic Particles: Anticipating Application to the Coolants in the Fusion Reactor
In the future fusion reactor we will use deuterium and tritium (both are isotopes of hydrogen) as fuel, although tritium is almost non-existent in nature. Thus, we will generate tritium in the fusion reactor by making neutrons react with the atom called lithium, which is familiar due to its use in batteries. Tritium that has been generated will be temporarily carried to the tritium-recovery equipment, and then will be injected into the plasma as fuel. In order to generate electricity, using a coolant we remove the heat from the fusion reactor. It is then necessary to turn the steam turbine. Thus, using a coolant that includes lithium atoms, we are considering undertaking both the generation of tritium and its transport to the recovery equipment, and also the removal of the heat to outside the fusion reactor simultaneously.
The candidate fluid for achieving this idea is molten salt. That fluid is salt mainly containing lithium (lithium fluoride) that is mixed with other fluorides and thawed at a high temperature. This molten salt is safe because there is no combustive reaction even if it comes into contact with water or air. Further, in the fusion reactor the plasma is confined by the strong magnetic field. Because molten salt has high electrical resistivity, it has the characteristic of being difficult to decelerate even in the magnetic field, too. As a coolant, this is an appropriate characteristic for the efficient transport of heat to outside the fusion reactor. Conversely, molten salt has a low level of hydrogen solubility. As a result, there is the problem that it is difficult to transport tritium (the chemical qualities are the same as hydrogen) generated in the reaction with lithium together with the molten salt to the tritium-recovery equipment.
At the National Institute for Fusion Science, we posed the idea of raising the hydrogen solubility by mixing fine metal dust particles into the molten salt. We then moved forward with an experiment to test this idea. This time, we mixed titanium particles with the molten salt called FLiNaK (a combination of lithium, tritium, and potassium fluorides), and measured hydrogen solubility. This time, despite mixing but one gram of titanium and one kilogram of molten salt, we confirmed that hydrogen solubility increased by more than 1,000 times compared to pure molten salt. We then undertook an experiment of two different methods of mixing titanium particles (nano titanium dispersion and titanium powder dispersion). In both cases there appeared high hydrogen solubility.
Because titanium is a metal with high reactivity, this result will be applied to coolant in the fusion reactor because we can anticipate the suppression of pipe corrosion in the fusion reactor. The reaction of molten salt and neutrons produces hydrogen fluoride and free fluorine. This fluoride and fluorine have corrosive properties. However, these will quickly react with metallic particles that have mixed with molten salt, and will change into non-corrosive materials. Moreover, in the future fusion reactor, it will be necessary to remove the tritium that has been absorbed by metallic materials. For that, we are investigating the use of high-frequency electromagnetic waves. By selective heating of metallic particles by high frequency electromagnetic waves (the molten salt is not heated), we are confident that we can effectively remove the tritium. Further, we also are moving forward with experiments utilizing hydrogen at the National Institute for Fusion Science. Please look forward to our next advance in research.
Figure.
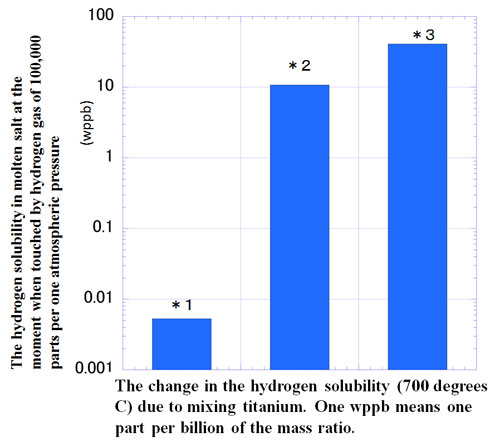
*1: Pure molten salt
*2: Molten salt with nano-titanium dissolved (the middle bar in the chart) is produced through a unique electrolysis method, that is, through dispersion of titanium particles below one micrometer (1/1000 millimeter) directly generated in the molten salt that does not come into contact with air.
*3: Molten salt with titanium powder dissolved (the right bar in the image) is produced through dispersion of titanium powders smaller than 38 micrometers.
